Abstract

Background: Sickle cell disease (SCD) pain is associated with colder temperatures, touch and increased wind speed suggesting thermal and mechanical pain nociceptors may be activated during and/or precipitate acute SCD pain. Patients with SCD and SCD mice display features of nervous system sensitization during baseline health as evidenced by hypersensitivity to thermal and mechanical pain. In SCD mice, this pain hypersensitivity is exacerbated with hypoxia-reoxygenation that mimics acute vaso-occlusive crises. However, it is unknown if exacerbations of thermal and mechanical pain hypersensitivity occur during acute pain in SCD patients. Thus, we sought to quantify thermal and mechanical pain sensitivity differences between SCD patients in baseline health and during pain. We hypothesized SCD patients will experience increased hypersensitivity to thermal and mechanical pain during acute pain as compared to baseline health.
Methods: We conducted a prospective cohort study of SCD patients ≥ 7 yrs assessed in baseline health and during hospitalization for pain to detect patients' sensitivity to thermal and mechanical stimuli as measured by quantitative sensory testing (QST). We excluded patients with pain phenotype other than SCD, known overt/silent stroke or those chronically transfused. Patients underwent QST to thermal (cold/heat) and mechanical stimuli during baseline health and within 48 hrs of hospitalization for pain. QST detects sensory loss (hyposensitivity) or gain (hypersensitivity). Thermal testing was done with a Thermal Sensory Analyzer (Medoc;Israel), a computer-assisted device that delivers cold/warm stimuli via a skin thermode (starting temperature, 32ºC; range, 0-50ºC). Mechanical testing was done using graded vonFrey filaments (force range 0.026-110 g). Testing sites were the thenar eminence of non-dominant hand (glabrous skin) and lateral dorsum of foot (hairy skin). Primary outcomes were: 1) Cold Pain Threshold (ºC), 2) Heat Pain Threshold (ºC) and 3) Mechanical Pain Threshold (g) assessed by "method of limits" where patients pushed a button (thermal) or spoke (mechanical) when the progressive stimulus was painful. The final outcome was the median of 3 (thermal) and 5 (mechanical) tests. Patient-report of pain precipitators related to temperature was collected. Descriptive statistics were performed and comparison of medians between baseline and pain was done with Wilcoxon-signed rank due to non-normally distributed data. Linear effect model with repeated measure analysis was done to adjust for age, gender and hydroxyurea use. Statistical significance was set at p<0.05.
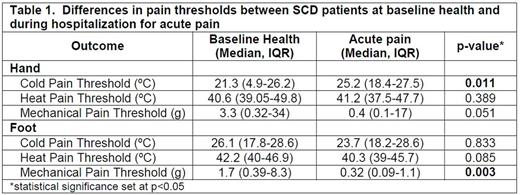
Results: We enrolled 24 SCD patients that were tested both in baseline health and when hospitalized for pain. The median age of baseline testing was 10.5 (IQR 9-14.8) yrs, 66.7% were female and 91.7% were on hydroxyurea. Median time between baseline and pain testing was 15.8 (IQR 5.1-22.1) mos. Genotypes were 62.5% HbSS, 29.2% HbSC, 4.2% HbSβ+thal, and 4.2% HbSOArab. SCD patients had significantly increased cold pain sensitivity in the hand during pain as compared to baseline health and also had significantly increased mechanical pain sensitivity in the foot during pain as compared to baseline health (Table 1). There were no differences in heat pain sensitivity during pain (Table 1). Significant changes in cold (1.6 ºC, p=0.03) and mechanical (-6.6g, p=0.002) pain sensitivity persisted after adjusting for age, gender and hydroxyurea use. Significantly more patients reported temperature change from warm to cold exacerbates their pain as compared to change from cold to warm (63.2% vs. 26.3%, p=0.03; McNemar Test).
Conclusions: SCD patients experience exacerbations of cold and mechanical pain sensitivity during hospitalization for pain, however, no changes in heat pain sensitivity. This differential pattern is consistent with patient reports of cold temperatures exacerbating pain, hypersensitivity to touch during pain events and heat providing pain relief. The exacerbation of mechanical and cold pain emulate findings in the SCD mouse model and suggest cold and mechanical pain nociceptors may contribute to SCD pain biology. Modulation of these pathways could be novel therapeutic targets to treat and/or prevent SCD pain. Investigation into why these pain nociceptors are activated in SCD patients could lead to increased understanding of SCD pain biology and additional therapeutic targets.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal